In routine clinical practice, comprehensive genomic profiling analysis using targeted next-generation sequencing (NGS) panels plays a crucial role in guiding treatment decisions for patients in advanced stages. However, the poor quality of genetic material extracted from FFPE tissue blocks may impact the reliability of NGS data (Sah et al, 2013; Kim et al., 2017; Cucco et al., 2018).
Among the different pre-analytical steps involved in the generation of FFPE tissue blocks, the most critical one is represented by Formalin fixation. Even following the standard use of Neutral Buffered Formalin (NBF), extensive DNA fragmentation and chemically induced changes are often affecting the results.
The innovative fixative developed by ADDAX Biosciences has been shown to radically improve DNA quality and reduce artefacts compared to Formalin-fixed tissues. GAF allows for improved genomic analysis of tissues and enables broader and more complete profiling.

GAF® overcomes the artifacts and low DNA preservation observed in Formalin fixation.
The study, published in the journal Laboratory Investigation, considered several alternatives to traditional Formalin-containing fixatives. The quality of genetic material that could be extracted from samples fixed with neutral-buffered Formalin (NBF) and acid-free fixatives (Glyoxal Acid-Free – GAF) was examined.
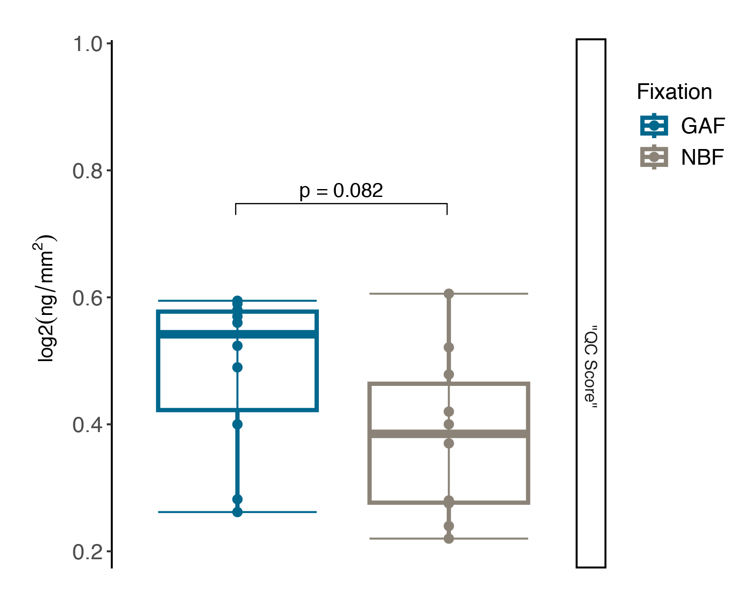
DNA Integrity number (Quality control for DNA fragmentation)
Parameter: DNA Integrity Number (Tapestation)
Result: significant impact of GAF fixation type over DIN compared to NBF
Among these fixatives, NBF resulted as the lowest performance in terms of DNA preservation and sequencing in respect to acid deprived fixatives.
Fixation in phosphates buffered Formalin (PBF) causes multiple artefacts, most notably cytosine deamination in uracil and widespread DNA fragmentation.
The study demonstrates that GAF® outperforms Formalin in terms of DNA quality (greater fragment length), leading to reduced false-positive results for variants.
GAF® stood out as the only non-carcinogenic option that overcomes the artifacts and low DNA preservation observed in Formalin fixation.
Extract from the study “Alternative protocols of tissue fixation dramatically reduces the impact of DNA artifacts, unraveling the interpretation of clinical Comprehensive Genomic Profiling”.
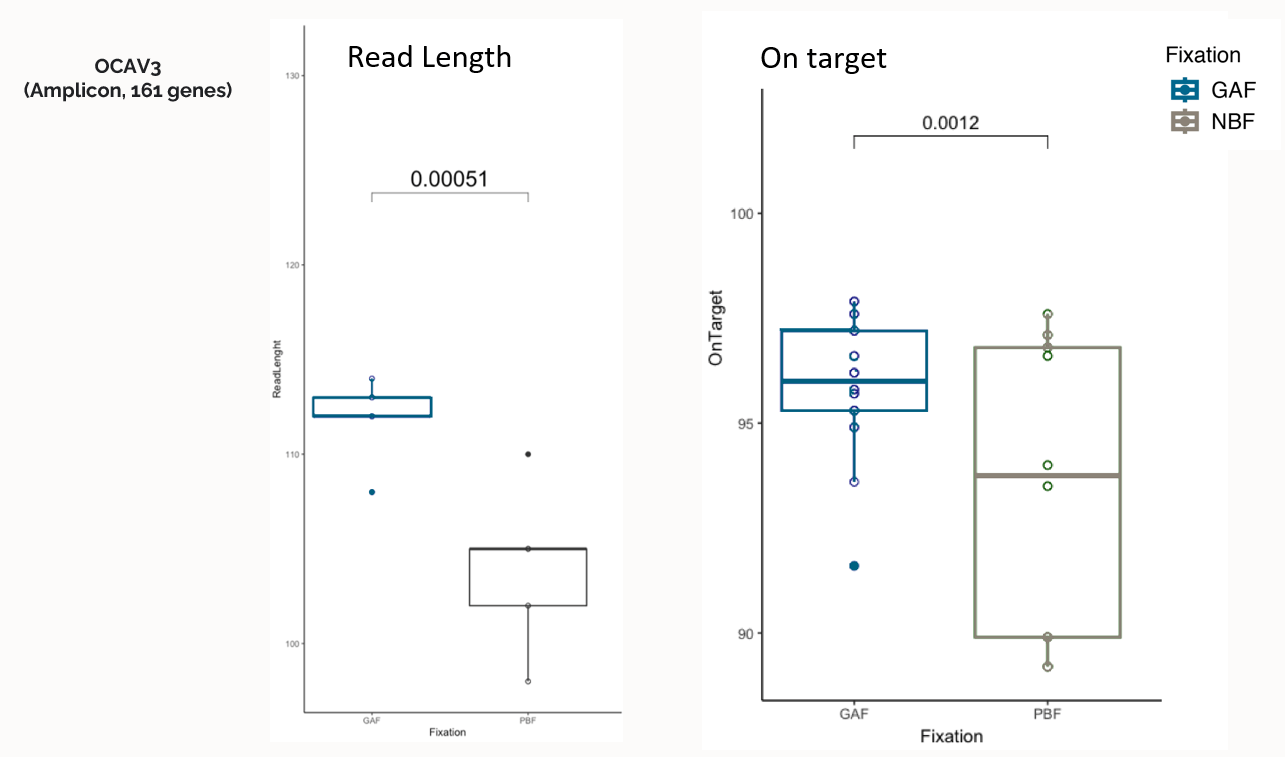
Significant impact of GAF fixation on read length and on target sequencing
In precision oncology, molecular diagnostics is based on the identification of a specific alteration for the administration of a therapeutic compound that is believed to be the most precise in addressing the disease. However, even diseases with robust biomarker tests can suffer from a lack of precision. The use of Formalin-fixed paraffin-embedded (FFPE) samples often hamper the extraction of high-quality genetic material, which can compromise the reliability of sequencing data.
Pre-analytical handling of tissues in oncology (but not only) is crucial for successful molecular diagnostic testing. Fixation in phosphates buffered Formalin (PBF) causes multiple artefacts and hampers the extraction of high-quality genetic material, which can compromise the reliability of sequencing data.
Through the validation study, we have demonstrated how a fixative such as glyoxal, a dialdehyde with similar reactivity properties to Formalin, deprived of glyoxylic acid (GAF), succeeds in reducing the artefactual effects of Formalin fixation.
PRE-ANALITICS:
Qualitative DNA parameters for QCs were significantly influenced by fixation type with increase quality using GAF in respect to PBF.
ONCOSIGNATURE:
OCAV3 QCs identified an increased read quality and a reduced fragmentation correlated with the quality identified in the pre-analytics.
Today, sequencing tumors only identifies a genomic alteration in 50% of cases, averagely. The goal is to achieve much higher percentages. Currently, a significant number of samples may be unsuitable for molecular analysis due to poor quality of nucleic acids. The low quality of genetic material extracted from Formalin-fixed tissue blocks greatly impacts data reliability. DNA fragmentation and chemical changes induced by Formalin often influence the results.
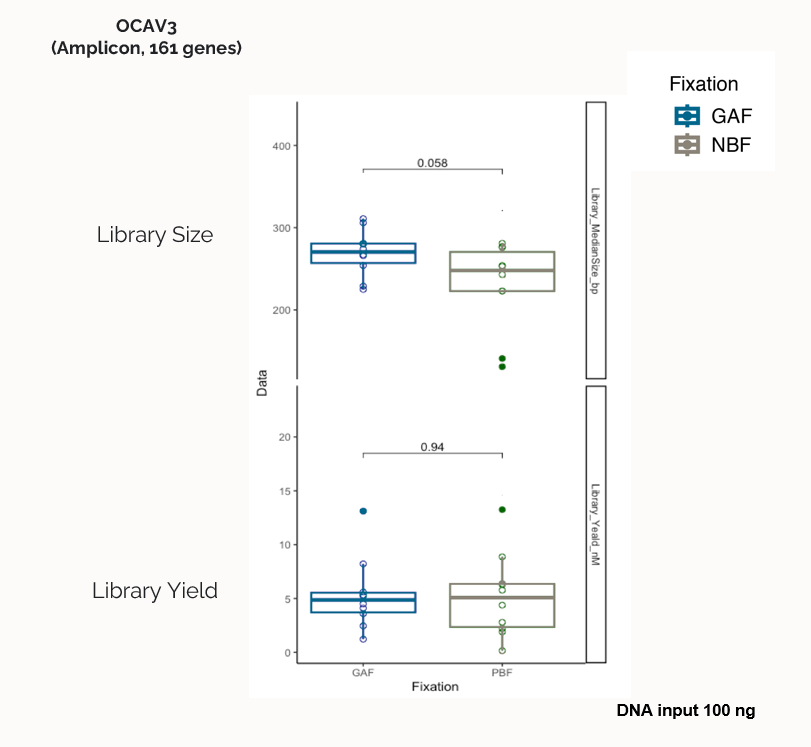
Significant impact of PBF fixation type over library median size, with reduced size compared to GAF.
No impact of fixation type over library yield.
The use of acid-deprived fixatives opens up the possibility of implementing more complex molecular profiling techniques on tissue samples. This remarkable outcome represents a significant advancement for next-generation sequencing (NGS) panels, particularly when comprehensive genomic profiling analysis is essential for informed treatment decisions.
ADDAX Biosciences was born as a spin off of the University of Turin, which contribuited with support to the research activity.

Our company has received funding from the European Union’s Horizon 2020 Research and Innovation Programme under Grant Agreement No 8157692.
The project “GAF – Towards a hospital without Formalin” is realized thanks to the co-financing of POR FESR Piemonte 2014-2020, Ob.1 – “Research, technological development and innovation, I.1.b.4.1 “Support for creation and consolidation of innovative start-ups with a high intensity of application and knowledge and research spin-off initiatives”.
The project aims to validate, produce and market GAF, a histological fixative without carcinogenicity, that works as a substitute to Formalin. Achieving the project’s objectives will improve the working conditions of health and research professionals, protecting them from the risk of developing occupational diseases caused by inhaling Formalin vapours.